∆G°=-Rtlnk
∆G°=-Rtlnk. In classes i've taken the equation is just given to us without derivation and i haven't been able to find any clear answers/derivations. = ccравн ×dd равн aaравн ×bbравн. In addition ∆g is unaffected by external factors that change the kinetics of the reaction. By definition delta g is going to be the same as delta go under standard conditions. • changes in h an s during a reaction result in a change in free energy, ∆g , given by the equation. Equation relating change of gibbs free energy to standardized change of gibbs free energy. At any stage of the reaction, the gibbs free energy and the gibbs free energy(standard ) are related by the equation.
In addition ∆g is unaffected by external factors that change the kinetics of the reaction. • thus, if you can show that ∆g is negative at a given temperature and pressure, you can predict that the reaction will be spontaneous. Here is the link to the information about the question. Equation relating change of gibbs free energy to standardized change of gibbs free energy. Where ∆g is the difference in the energy between reactants and products. In classes i've taken the equation is just given to us without derivation and i haven't been able to find any clear answers/derivations.
Where q represents the reaction quotient.
By definition delta g is going to be the same as delta go under standard conditions. Equation relating change of gibbs free energy to standardized change of gibbs free energy. For example if e a (activation energy) were to decrease in the presence of a catalyst or the kinetic energy of molecules increases due to a rise in. In addition ∆g is unaffected by external factors that change the kinetics of the reaction. K is the equilibrium constant, meaning it is products divided by reactants when. In thermodynamics, the gibbs free energy (or gibbs energy) is a thermodynamic potential that can be used to calculate the maximum reversible work that may be performed by a thermodynamic system at a constant temperature and pressure. I need help finishing the problem. Starting with the change in free energy at constant temperature: • thus, if you can show that ∆g is negative at a given temperature and pressure, you can predict that the reaction will be spontaneous. = ccравн ×dd равн aaравн ×bbравн. The classical carnot heat engine. See more videos of delta g equals rtlnk. Where ∆g is the difference in the energy between reactants and products. • changes in h an s during a reaction result in a change in free energy, ∆g , given by the equation. At any stage of the reaction, the gibbs free energy and the gibbs free energy(standard ) are related by the equation.
See more videos of delta g equals rtlnk. At any stage of the reaction, the gibbs free energy and the gibbs free energy(standard ) are related by the equation. Where q represents the reaction quotient. In classes i've taken the equation is just given to us without derivation and i haven't been able to find any clear answers/derivations. Starting with the change in free energy at constant temperature: The classical carnot heat engine. Equation relating change of gibbs free energy to standardized change of gibbs free energy. In addition ∆g is unaffected by external factors that change the kinetics of the reaction.

Equation relating change of gibbs free energy to standardized change of gibbs free energy.
In thermodynamics, the gibbs free energy (or gibbs energy) is a thermodynamic potential that can be used to calculate the maximum reversible work that may be performed by a thermodynamic system at a constant temperature and pressure. This video took me weeks to do, calling friends and reading the text book i used as a kid. Equation relating change of gibbs free energy to standardized change of gibbs free energy. At any stage of the reaction, the gibbs free energy and the gibbs free energy(standard ) are related by the equation. Here is the link to the information about the question. • thus, if you can show that ∆g is negative at a given temperature and pressure, you can predict that the reaction will be spontaneous. By definition delta g is going to be the same as delta go under standard conditions. K is the equilibrium constant, meaning it is products divided by reactants when. Starting with the change in free energy at constant temperature: In addition ∆g is unaffected by external factors that change the kinetics of the reaction.
= ccравн ×dd равн aaравн ×bbравн. Starting with the change in free energy at constant temperature: Here is the link to the information about the question.
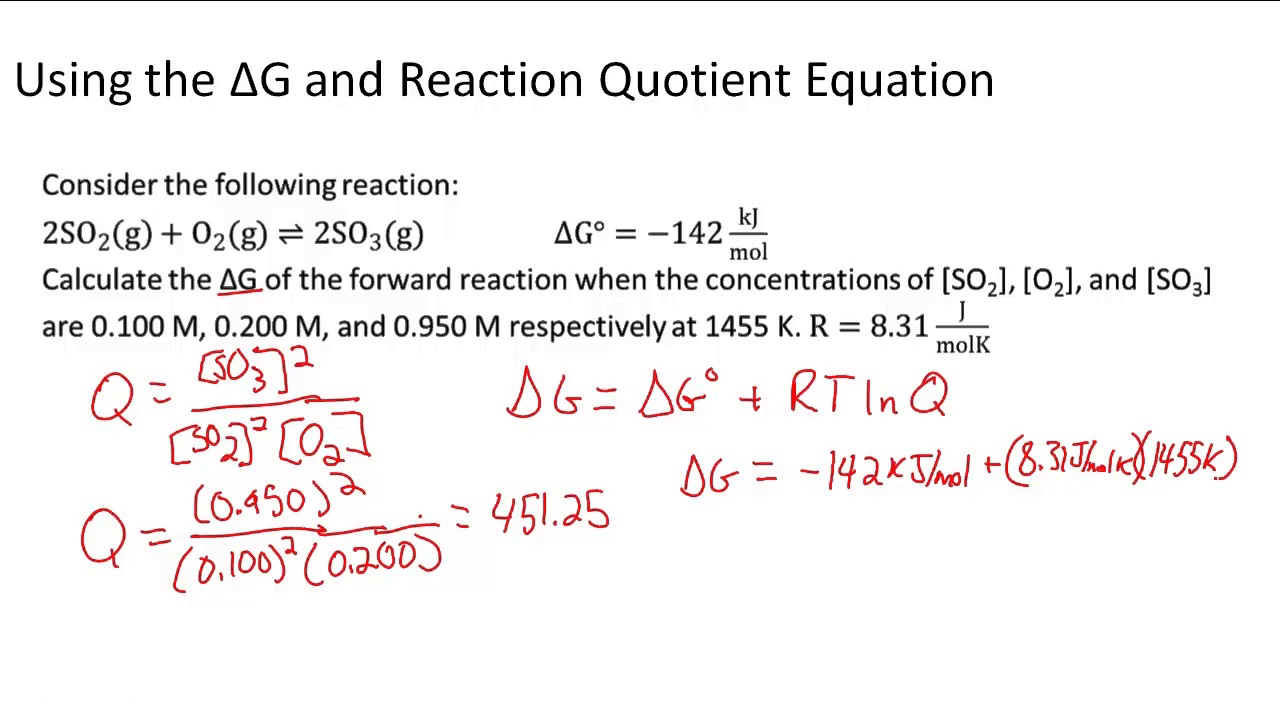
This video took me weeks to do, calling friends and reading the text book i used as a kid.
= ccравн ×dd равн aaравн ×bbравн. In classes i've taken the equation is just given to us without derivation and i haven't been able to find any clear answers/derivations. For example if e a (activation energy) were to decrease in the presence of a catalyst or the kinetic energy of molecules increases due to a rise in. Starting with the change in free energy at constant temperature: Here is the link to the information about the question. Where ∆g is the difference in the energy between reactants and products. By definition delta g is going to be the same as delta go under standard conditions. In thermodynamics, the gibbs free energy (or gibbs energy) is a thermodynamic potential that can be used to calculate the maximum reversible work that may be performed by a thermodynamic system at a constant temperature and pressure. Contribute to manulove/onetapapi development by creating an account on github. K is the equilibrium constant, meaning it is products divided by reactants when. Where q represents the reaction quotient.
By definition delta g is going to be the same as delta go under standard conditions rtlnk. The classical carnot heat engine.
 Source: www.life.illinois.edu
Source: www.life.illinois.edu For example if e a (activation energy) were to decrease in the presence of a catalyst or the kinetic energy of molecules increases due to a rise in.
 Source: images.slideplayer.com
Source: images.slideplayer.com In thermodynamics, the gibbs free energy (or gibbs energy) is a thermodynamic potential that can be used to calculate the maximum reversible work that may be performed by a thermodynamic system at a constant temperature and pressure.
 Source: www.researchgate.net
Source: www.researchgate.net Here is the link to the information about the question.
 Source: quizlet.com
Source: quizlet.com At any stage of the reaction, the gibbs free energy and the gibbs free energy(standard ) are related by the equation.
 Source: www.researchgate.net
Source: www.researchgate.net K is the equilibrium constant, meaning it is products divided by reactants when.
 Source: i.ytimg.com
Source: i.ytimg.com This video took me weeks to do, calling friends and reading the text book i used as a kid.
 Source: media-temporary.preziusercontent.com
Source: media-temporary.preziusercontent.com In thermodynamics, the gibbs free energy (or gibbs energy) is a thermodynamic potential that can be used to calculate the maximum reversible work that may be performed by a thermodynamic system at a constant temperature and pressure.
 Source: images.slideplayer.com
Source: images.slideplayer.com Equation relating change of gibbs free energy to standardized change of gibbs free energy.
 Source: images.slideplayer.com
Source: images.slideplayer.com I need help finishing the problem.
 Source: study.com
Source: study.com See more videos of delta g equals rtlnk.
 Source: www.life.illinois.edu
Source: www.life.illinois.edu K is the equilibrium constant, meaning it is products divided by reactants when.
 Source: chem.libretexts.org
Source: chem.libretexts.org For example if e a (activation energy) were to decrease in the presence of a catalyst or the kinetic energy of molecules increases due to a rise in.
 Source: s2.studylib.net
Source: s2.studylib.net K is the equilibrium constant, meaning it is products divided by reactants when.
In addition ∆g is unaffected by external factors that change the kinetics of the reaction.
 Source: s3.studylib.net
Source: s3.studylib.net In classes i've taken the equation is just given to us without derivation and i haven't been able to find any clear answers/derivations.
 Source: prod-qna-question-images.s3.amazonaws.com
Source: prod-qna-question-images.s3.amazonaws.com K is the equilibrium constant, meaning it is products divided by reactants when.
In classes i've taken the equation is just given to us without derivation and i haven't been able to find any clear answers/derivations.
 Source: www.researchgate.net
Source: www.researchgate.net Starting with the change in free energy at constant temperature:
 Source: qph.fs.quoracdn.net
Source: qph.fs.quoracdn.net = ccравн ×dd равн aaравн ×bbравн.
 Source: upload.wikimedia.org
Source: upload.wikimedia.org See more videos of delta g equals rtlnk.
 Source: www.researchgate.net
Source: www.researchgate.net Here is the link to the information about the question.
Here is the link to the information about the question.
 Source: www.calctool.org
Source: www.calctool.org Starting with the change in free energy at constant temperature:
Here is the link to the information about the question.
 Source: www.coursehero.com
Source: www.coursehero.com Here is the link to the information about the question.
Where ∆g is the difference in the energy between reactants and products.
 Source: d2vlcm61l7u1fs.cloudfront.net
Source: d2vlcm61l7u1fs.cloudfront.net Starting with the change in free energy at constant temperature:
 Source: images.slideplayer.com
Source: images.slideplayer.com The classical carnot heat engine.
 Source: images.slideplayer.com
Source: images.slideplayer.com This video took me weeks to do, calling friends and reading the text book i used as a kid.
 Source: www.coursehero.com
Source: www.coursehero.com By definition delta g is going to be the same as delta go under standard conditions.
 Source: www.chemicalforums.com
Source: www.chemicalforums.com In classes i've taken the equation is just given to us without derivation and i haven't been able to find any clear answers/derivations.
By definition delta g is going to be the same as delta go under standard conditions.
 Source: s2.studylib.net
Source: s2.studylib.net • changes in h an s during a reaction result in a change in free energy, ∆g , given by the equation.
 Source: cf.ppt-online.org
Source: cf.ppt-online.org In classes i've taken the equation is just given to us without derivation and i haven't been able to find any clear answers/derivations.
Posting Komentar untuk "∆G°=-Rtlnk"